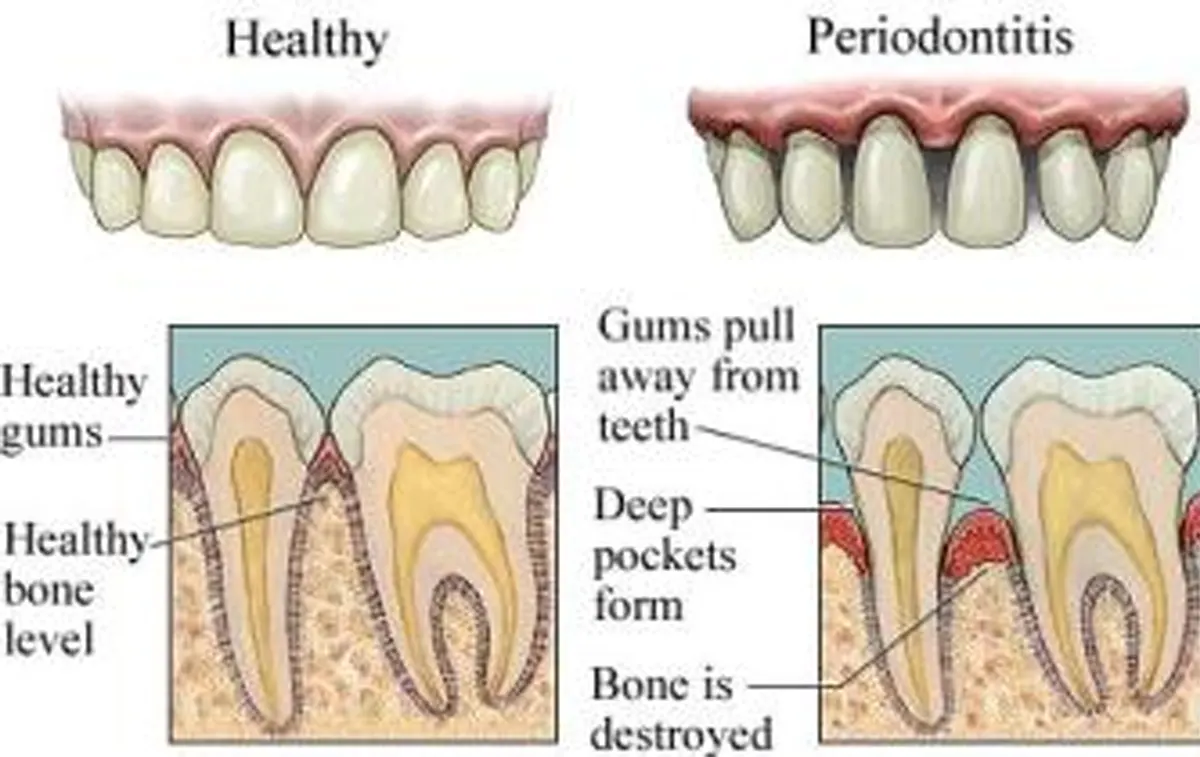
The etiology of the periodontal disease is multifactorial, but bacterial plaque is necessary tor the initiation and progress kin of inflammatory periodontal disease. As indicated by the new Classification System for periodontal disease and Conditions, etiologic factors have become the framework for periodontal diagnosis and classification
What Is Inflammation?
Inflammation is a soft tissue cellular and vascular response to local injury of physical, thermal, chemical, or microbial origin. Inflammatory periodontal disease are no exception to this paradigm as local periodontal etiologic factors may be physical (factitial habits such as toothbrush abrasion or occlusal trauma), thermal, chemical (epithelial disorders associated with some mouthwashes, smokeless tobacco, aspirin, and cocaine), and microbial (dental plaque induced gingival diseases). The most common inflammatory periodontal disease are caused by a local accumulation of bacteria.
What Is Meant by Etiology?
Etiology is “the study or theory of the causation of any disease; the sum of knowledge regarding causes.” Therefore, etiology is a noun that detines the science of disease causation, but in common usage, etiology is a cluster of factors that contribute to disease (ie, the etiology of periodontal disease).
What Are the Microbiologic Etiologic Factors in Periodontal Diseases?
The microbiologic etiologic factor in periodontal disease is dental plaque with dental calculus as probably the most significant local contributing factor. Food debris and the bacteria it contains is probably a major etiologic factor in root carts.
There is little dispute over the concept that bacteria are the fernery etiologic factors in inflammatory periodontal disease in 1965, Loe and co-workers published their classic work that demonstrated that gingival health could be reliably achieved with immaculate oral hygiene and that gingival inflammation could be caused by the accumulation of plaque on the teeth.
Light microscopic examination of tooth scrapings revealed that plaque was an adherent mat of bacteria, epithelial cells, and leukocytes encased in an amorphous protein and polysaccharide matrix, and that cocci, filamentous bacteria, spirochetes, and vibrios accumulated on teeth in an ordered sequence. The knowledge produced in this and later studies of periodontal disease and plaque morphology and microbiology emphasied that plaque was a heterogeneous community of bacteria.
Generally speaking, bacteria associated with periodontal health are characterized as Gram-positive, nonmotile facultative anaerobes. Bacteria associated with periodontal disease are generally Gram-negative, motile, strictly anaerobic species. The cell wall of Gram-negative bacteria consists of a lipopolysaccharide base, also known as endotoxin that has significant pathogenic potential. While over 350 distinct species of bacteria have been isolated from the oral cavity, relatively few are associated with gingival or periodontal inflammation. The list of strongly associated pathogenic bacteria includes:
Actinobacillus actinomycetemcomitans, Bacteroides forsythus, Fusobacterium nucleatum, Peptostreptococcus micros, Porphyromonas gingivalis and Pre vote/la intermedia/nigresens
What Is Meant by the Term “Biofilm”?
Bioflims form on inert surfaces where bacteria to bacteria cohesive interactions in periodontal disease or bacteria to surface adhesive interactions are allowed to occur. Biofilms are heterogeneous composites of bacterial communities within a nonbacterial protein, polysaccharide, and glycoprotein matrix of bacterial and salivary origin. The matrix allows for a circulation of nutrients and bacterial metabolites between communities and the environment outside the biofilm. There are extreme variations in oxygen levels ranging from highly aerobic areas within fluid channels to almost completely anaerobic areas in microcolonies.
What Is the “Nonspecific Plaque Hypothesis”?
The basic tenets of the nonspecific plaque hypothesis state that inflammatory periodontal disease (and possibly caries) are caused by composite effect of bacterial colonization and maturation on the surfaces of teeth, not by specific bacteria themselves. Gingival disease is the outcome from release of bacterial metabolites (such as butyrate or other short chain fatty acids) and immunogenic bacterial antigen components, such as lipopolysaccharide (endotoxin) from Gram-negative cell walls during plaque growth. Inflammatory periodontal disease is the outcome of a microbial mass that is in excess of the local defense mechanisms of the host.
What Is the “Specific Plaque Hypothesis”?
The specific plaque hypothesis states that periodontitis is an infection caused by a limited number of periodontal microorganisms and that these microorganisms characterize the plaque biotilms associated with periodontitis but not gingivitis or gingival health. It appears that of the 300+ identifiable species found in the oral cavity, only a small proportion (10- 12 species) are actually found in active periodontitis sites.
The bacteria believed to be pathogens in periodontal disease do not conform to the classic dogma for microbial pathogenicity (ie, Kochs Postulates). The current understanding of mixed infections, bacterial invasion, virulence factors, conducive bacteria habitats, the role of so-called beneficial species, and the susceptibility of the host have rendered Kochs Postulates obsolete when it comes to periodontitis.
What Is Meant by “Putative Periodontal Pathogen”?
The criteria for implicating oral microorganisms as periodontal pathogens are:
1. The microorganism must be associated in high numbers in active periodontal disease lesions and either absent (not cultivable) or in low numbers in gingivitis or healthy sites. The numbers of the microorganism should have increased to a threshold level before the onset of disease.
2. The elimination of the microorganism, or its numerical reduction below threshold levels, should parallel remission of active periodontal disease.
3. There should be a specific host immune response against the organism (i.e., elevated serum, salivary, and crevicular fluid antibody titers).
4. The microorganism should evoke virulence factors that contribute to its pathogenicity or explain periodontal disease pathobiology
5. The microorganism should produce periodontitis in animal model systems.
What Are the Systemic Diseases and War Conditions That Are Contributing Factors for Periodontal Disease?
Aside from the medications that affect the clinical presentation of plaque-induced gingival diseases (nifedipine for control of hypertension, phenytoin for control of epileptic seizures, and cyclosporine to control organ transplant Selection), most systemic diseases and conditions that may affect periodontal disease generally alter host barrier and host defense mechanisms.
The impact of diminished host susceptibility, along with the diverse virulence mechanisms invading microorganisms possess, help to explain the individual variations in periodontal disease patterns we see in systemically ill periodontal patients. An assessment of systemic contributions to periodontal diseases in our patients is critical to periodontal diagnosis and/or treatment planning.
The systemic diseases and conditions that commonly affect periodontal disease are: Uncontrolled type I and type II diabetes mellitus, HIV/AIDS, hormone imbalances, genetic predisposition, medications, smoking, and malnutrition.
1. Diabetes mellitus in periodontal disease. Diabetes mellitus (DM) is affecting a growing number of Americans. The incidence of the disease seems to vary according to ethnic origin, but it is estimated that 5% to 10% of individuals in the United States are affected with diabetes. DM is an aberration in Carbohydrate, lipid, and protein metabolism. Most of the morbid complications of DM stem from long-term impaired glucose metabolism. The characteristic hyperglycemia of uncontrolled DM is the basis for most of the vascular, cellular, and immune changes associated with the periodontal disease.
Epidemiologic data has made clear associations between increased severity of periodontal disease and uncontrolled type I and type II diabetes mellitus. Type I and type II uncontrolled diabetics tend to present with more gingival inflammation, more loss of periodontal attachment, and radiographic evidence of more bone loss than controlled or non diabetic individuals. There is agreement that periodontal disease patients whose DM is well controlled may receive periodontal therapy without restrictions, including periodontal surgery and implant placement.
Uncontrolled diabetics, poorly controlled diabetics, or diabetics whose control is unknown should only receive emergency periodontal therapy, and that treatment should be performed with intraprocedural and/or postoperative antibiotic coverage. The patient’s physician may also prescribe insulin or other antihyperglycemic agents to help limit post-operative infections or complications in wound healing.
2. HIV/AIDS. Given the immunosuppressed state of these individuals (decreased CD4 lymphocytes), an expectation for severe periodontal disease in patients with HIV/AIDS is reasonable. Indeed, these individuals suffer from other bacterial, viral, and fungal diseases more than those without HIV infection. Many succumb to these infections. Early studies of the periodontal status in AIDS patients indicated that these individuals showed increased severity of periodontal disease. HIV-gingivitis (linear erythema) and HIVperiodontitis (necrotizing ulcerative periodontitis) categories of periodontal diseases were quickly proposed to designate the unique clinical characteristics of periodontal diseases in this group. Recently, the issue has been challenged by those who report no increases in the prevalence or extent of periodontal diseases among HIV-positive individuals.
3. Smoking. Due to the vasoconstrictor effect of nicotine and the paralysis by carbon monoxide on the ability of hemoglobin to transport oxygen, it is understandable that smoking is a serious environmental risk factor for periodontal disease. The length of time an individual has been smoking and the frequency of smoking play contributory roles in the severity of periodontal disease in smokers. Smokers also have a greater accumulation of plaque and calculus than nonsmokers and may be more at risk to harbor periodontal pathogens.
While probing depth reduction following conventional nonsurgical and surgical periodontal therapy has been reported in smokers, the amount of reduction has been reported as less than that achieved in nonsmokers. A growing body of evidence suggests strongly that the failure rate of implant therapy is higher in patients who smoke. It is not uncommon for a therapist to recommend against the placement of dental implants in smokers. Patients must be counseled for periodontal disease and supported in their attempts to overcome their addiction.
4. Sex hormone imbalances. The most notable changes in the periodontium that are affected in part by hormonal changes occur in women in their childbearing years. In the case of pregnancy, progesterone and estrogen levels increase to levels that are several orders of magnitude greater than those seen during a normal menstrual cycle. Varying degrees of reversible pregnancy gingivitis are common during pregnancy. The biologic impact of hormone changes range from the release of periodontal disease inflammatory mediators that increase vascular permeability (prostaglandins), the alterations in immunoregulation and pro-inflammatory regulators, the imbalances in the fibrinolytic system, and the abundant growth of the periodontal disease pathogen, P. intermedia. Because the duration of pregnancy is relatively short, hormonal changes associated with pregnancy have little effect on the more irreversible progress of periodontitis.
Oral contraceptives mimic the hormonal levels seen during pregnancy, and it is not uncommon to find pregnancy-like changes in patients using birth control pills (BCP). Because gingival sex hormone concentrations tend to be lower during normal menstruation, it is not unexpected that women in their childbearing years may present with “cyclic” episodes of increased periodontal disease inflammation.
The most important concern of the dentist in managing patients who present with gingival disease related to hormone imbalances is to be certain that inflammatory periodontal disease control measures are effective.
This is particularly important in women who are pregnant because data exists to suggest a relationship between periodontal disease infections (periodontitis) and preterm low birth weight babies. Antibiotics should be used only after a medical consultation in patients who are pregnant. Although controversial, there are reports of decreased effectiveness of oral contraceptives in individuals taking certain antibiotics. Individuals who are taking BCPs should be advised that the use of prescribed antibiotics such as tetracycline and some penicillin may interfere with the action of BCPs. To avoid unwanted pregnancy, these individuals should be so warned and use alternative methods of birth control while taking antibiotics.
5. Genetic predisposition for periodontal disease. There is general agreement that individual responses to plaque bacteria vary. It has been suggested that disease pattern variations could be based, in part, on underlying genetically based differences in immune function. Indeed, the association of:
a. Neutrophil receptor defects
b. Antibody responses (lgG2) to periodontal pathogens
c. Certain histocompatibility antigens (HLA)
d. Lymphocyte immune regulatory defects in patients with aggressive periodontitis add credibility to this concept.
Studies in twins indicate that many of the clinical variations seen in chronic periodontal disease can be attributed to individual genetic differences. Recent reports of genetic pleomorphism and the elevated production of pro-inflammatory mediators, such as IL-i , add another dimension to the impact genetic variations among individuals have on the patterns of chronic periodontal disease.